
Chief Medical Officer
CANDIDATE INFORMATION PACK
Momentum Clinical Research


Executive Summary
- Pivotal Australasian medical leadership role advancing clinical research excellence
- Drive strategic growth, clinical governance and innovation across a 20-site trans-Tasman network
- Shape the next generation of research leadership in a dynamic, growth-focused organisation
THE OPPORTUNITY
Momentum Clinical Research (MCR) is seeking an experienced and commercially astute Chief Medical Officer to lead its medical and scientific agenda across Australia and New Zealand. MCR is Australasia’s largest integrated clinical trial network, recently formed through the merger of Momentum Clinical Research and Pacific Clinical Research Network (PCRN). With more than 20 sites delivering trials from Phase I through to Phase IV, MCR combines scale, agility, and deep therapeutic expertise across a broad range of clinical areas.
This is an opportunity to join a fast-evolving organisation at a defining stage of its growth. MCR has a strong market presence, a flexible and entrepreneurial culture, and a clear vision to challenge industry norms and shape the future of clinical research delivery. The company is expanding its capability, reputation and reach, providing the successful candidate with an exciting platform to lead innovation, build high-performing teams and influence the next phase of trans-Tasman growth.
Based in Sydney at the Holdsworth House facility in Darlinghurst, the Chief Medical Officer will be part of an energetic and collaborative executive team. The role offers the appeal of contributing to a purpose-driven business that values clinical excellence, commercial innovation, and genuine connection with patients, investigators, and partners.
THE ROLE
Reporting to the Chief Executive Officer, the Chief Medical Officer provides strategic medical and scientific leadership. The role ensures clinical integrity, governance, and compliance while supporting growth and new business opportunities, and building key partnerships. As technical lead of the medical team and Principal Investigator on selected studies, the CMO will set the medical strategy, strengthen research quality, and contribute to business development initiatives that enhance MCR’s market position and long-term sustainability.
THE CANDIDATE
The ideal candidate will have specialist qualifications and substantial experience as a Clinical Trial Investigator or senior medical leader within research, healthcare, or the pharmaceutical sector. They will demonstrate a blend of clinical credibility, commercial insight, and leadership gravitas — capable of balancing scientific integrity with strategic and business imperatives. Strong stakeholder engagement skills, decisive judgement, and the ability to inspire confidence across clinical and corporate settings will be essential.
For a confidential discussion, please get in touch with:
Janine Hammat
HG Principal Consultant
M. +61 (0)488 555 858 E. jhammat@hardygroupintl.com

Momentum Clinical Research
Accelerating medical research with the largest network of fully integrated clinical trial sites across Australasia
At Momentum Clinical Research, we take pride not only in the quality and reliability of the work we deliver, but in how we deliver it. Our team is energised by the impact of clinical research, and that energy, personality, and genuine enjoyment infuse every partnership.
We believe the clinical trial journey can and should be both effective and enjoyable — for our research partners and our participants. It’s a privilege to contribute to the advancement of medicine, and we approach that responsibility with both gratitude and a strong sense of human connection.
Momentum Clinical Research (MCR), strengthened by its merger with Pacific Clinical Research Network (PCRN), is a 20-site network across Australia and New Zealand delivering world-class clinical trials from Phase I through Phase IV, including a dedicated Phase I unit. With expanded operational scale, integrated systems, and a growing team of highly skilled professionals, we are positioned as one of Australasia’s most capable and agile trial delivery platforms.
Our Values
Innovative Thinking - We are progressive, future-focused and innovatively minded.
Medical Excellence - We are science led, data driven and meticulous with detail.
Empathic Care - We listen with empathy, we are warm , approachable and easy to work with.
Thriving People - We are principled, people centered and ethically grounded.

Our Evolution
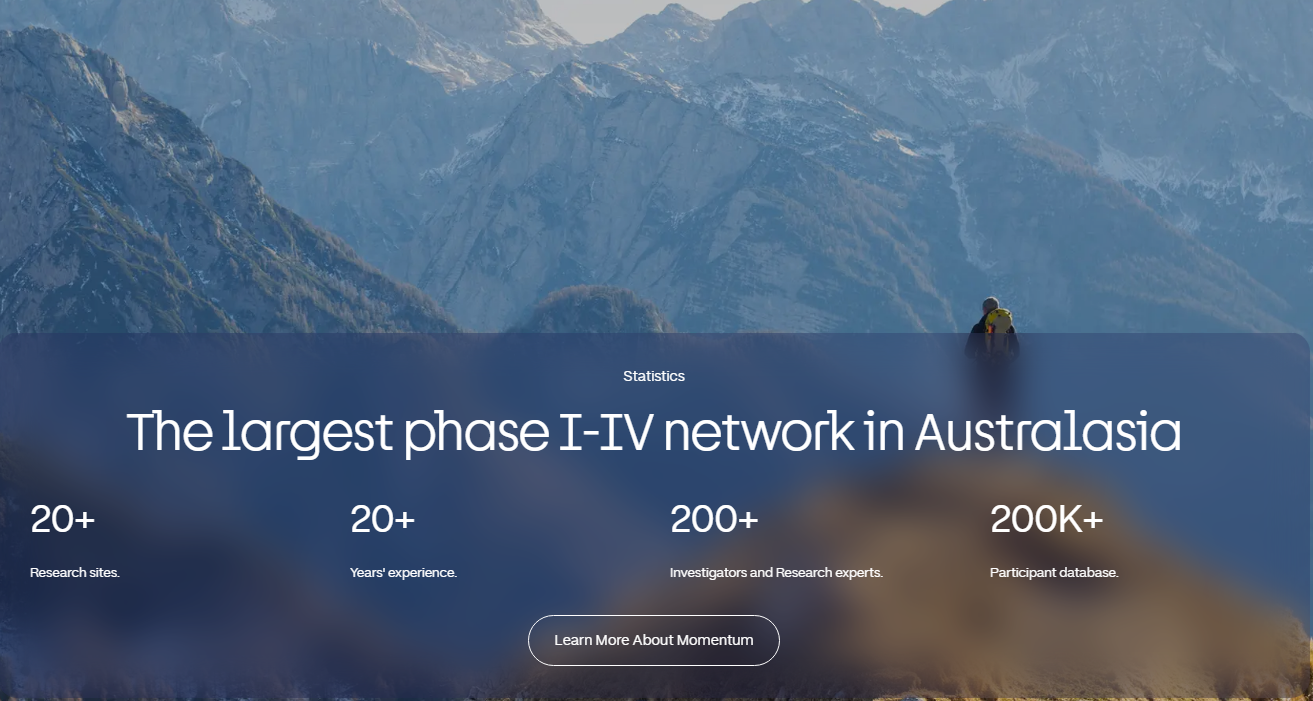
The largest network in Australasia.
Momentum Clinical Research has recently joined with PCRN to form Australia and New Zealand’s largest, most capable clinical trial network. Each of our founding companies contribute over two decades of expertise to create a foundation of knowledge, collaboration, and innovation in clinical research.
With more than 20 sites across the region, we deliver end-to-end trials from Phase 1 to Phase 4. By combining centralised efficiencies with strong local site leadership, we give sponsors faster, more reliable studies while expanding community access to innovative treatments.
What truly sets us apart, though, is the experience of working with us. We’re transparent, we follow through, and we make the process feel personal, positive, and engaging.
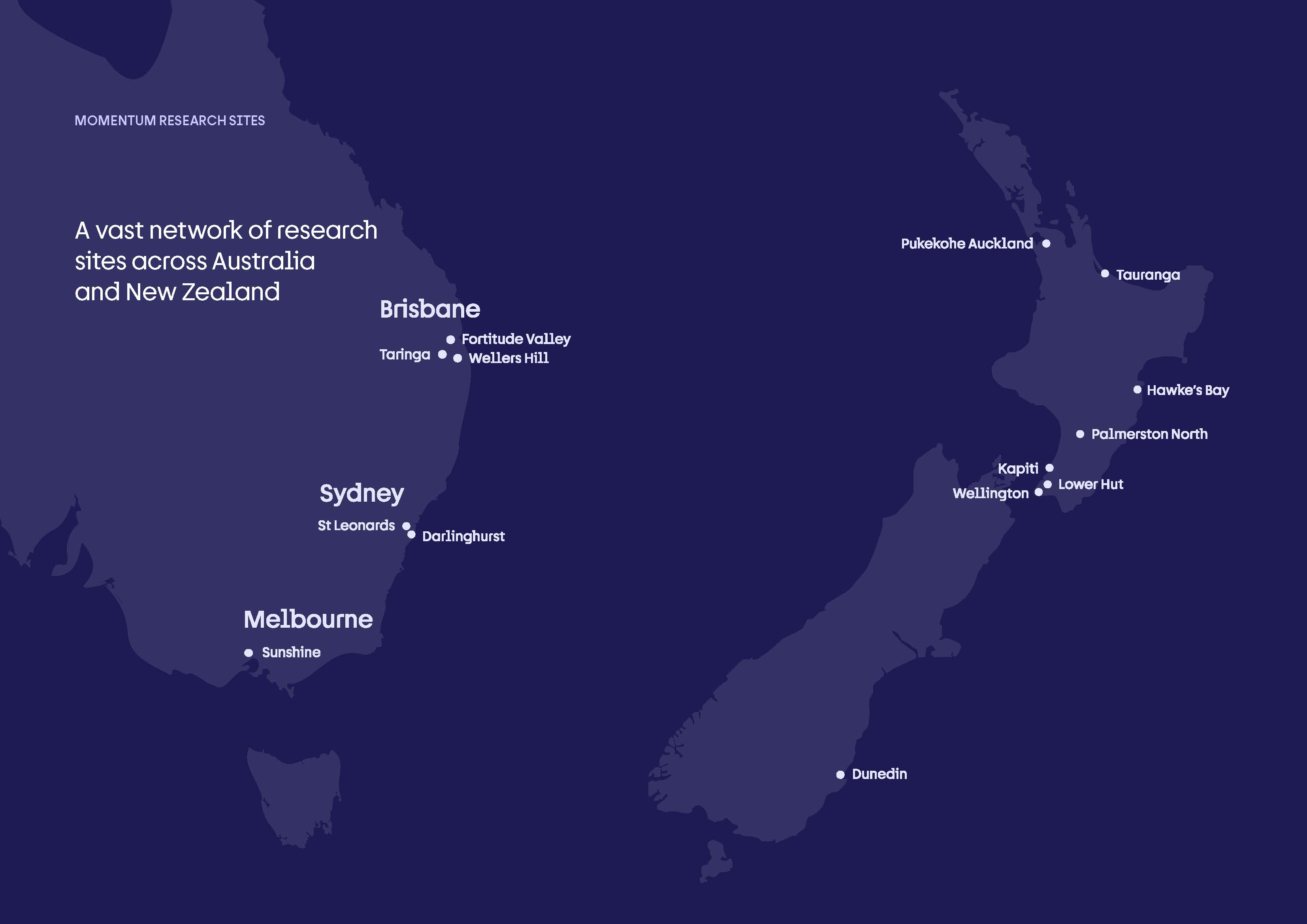
NB 6 PCRN Sites to be added post merger. See more about PCRN here.
Our State of the Art Facilities Provide
- Spacious clinics can accommodate multiple participants at a time
- Comfortable consultation rooms for enhanced participant experience
- Multi-purpose rooms adaptable to study requirements
- Special facilities for prolonged participant visits
- Private fully equipped examination rooms
- Fully secure and equipped laboratory and pharmacy rooms
- On-site laboratory for processing biologic samples
- RealTime CTMS platform to enable remote monitoring
- Secure controlled document storage for commercially sensitive and blinded information
- Space for onsite monitoring visits
- Easy access via public transport

Our Current Studies
- Rheumatology - Knee Osteoarthritis Studies
- Endocrinology & Metabolic Disorders, Cardiology - Obesity & Type 2 Diabetes Studies
- Dermatology - Alopecia Areata Studies
- Covid-19 Study
- Hyperlipidemia Studies
- Sexual Health, Urology Studies
- Respiratory Viruses Vaccine Studies
- Bronchiectasis Studies
- Chronic Cough Study
- COPD Study
- Vitiligo Study
- Chronic Kidney Disease & High Blood Pressure Studies
- Coeliac Disease Study
- Cardiovascular Study
- Migraine Study
- Shingles ZOSTER Vaccine Study
- Acne Studies
- Norovirus Studies
- Hives Studies
- Diabetes Studies
- HPV Studies
- Anxiety Studies
- Eczema (Atopic Dermatitis) Studies
- UTI Studies
- Panic Disorder Study
- RSV (Respiratory Syncytial Virus) Studies
- Asthma Studies
Find our current studies here.
Therapeutic Expertise
- General Medicine
- Respiratory medicine
- Endocrinology
- Cardiology
- Gastroenterology
- Dermatology
- Rheumatology
- Immunology and Allergies
- Men and Women’s Health
- Weight Management
- Diabetes and Metabolic
- Disorders, Coeliac Disease
- IBS
- Chronic Pain
- HIV
- Infectious Disease
- Mental health
- Lifestyle Diseases
- PK Studies
- Vaccine Development
- Healthy Participant Studies
Momentum case studies
At Momentum Clinical Research, success is measured by the impact of our work on global healthcare. Over the past 20 years, our clinical trials have contributed to the study data published in groundbreaking research papers and played a vital role in bringing life-changing medications to the global market.
Explore our research case studies to discover how our innovative approach to clinical trials can help scale your research across Australia and New Zealand. More about our success here.
Our Sponsors
Australasia's largest full phase clinical trial network
Introducing the people and processes that drive our uniquely integrated and relationship focused approach to delivering phase l-lV clinical trials. Our scale and centralised efficiency offer a distinct advantage to our partners, but it’s the experience of working with us that really sets us apart. We’re transparent, we follow through, and we make the process feel personal, positive and engaging.
Why sponsors and CROs worldwide partner with Momentum
- Accelerated Start Up
- Reliable Recruitment
- Streamlined and Integrated Process
- Diverse Expertise and Capabilities
- Highest Quality Data and Compliance
More about our world leading Pharmaceutical and Biotech companies partner here.
Role Specification
The Chief Medical Officer (CMO) provides strategic medical and scientific leadership across MCR, ensuring research integrity, clinical excellence, and alignment with business objectives. The role combines responsibility for advancing the organisation’s research and clinical agenda with a strong focus on identifying and developing new business opportunities, partnerships, and funding streams.
The CMO provides oversight of clinical and regulatory governance and builds trusted relationships with external stakeholders. In parallel, the CMO leverages their medical expertise and industry insight to drive business development, positioning the organisation as a leader in medical research and strengthening its commercial sustainability.
As technical lead of the medical team this role will also be assigned as Principal Investigator (PI) on specific studies (refer to Investigator position description).
Strategy
- Ensure medical strategy is aligned with MCR purpose, values, and stakeholder expectations.
- Engage with Board and/or leadership team and external stakeholders to refine and pressure-test strategic direction.
- Guide decision on resource allocation, capability development, and partnerships to enable strategic execution.
- Identify risks associated with strategic options and embeds mitigation planning into proposals.
- Provide expert advice to the executive team on clinical, scientific, and ethical issues.
- Drive innovation in clinical care, translational research, and new treatment approaches.
Leadership
- Develop, motivate, and inspire team to deliver high performance, engagement, and inclusion.
- Leads confidently through change, managing ambiguity and resistance while maintaining focus on outcomes.
- Onboard the Investigators and lead monthly investigator meetings.
- Develop Key performance indicators in line with Company strategy.
Clinical Governance & Quality
- Oversee the development and implementation of clinical governance frameworks, ensuring compliance with medical, ethical, and regulatory standards.
- Champion quality improvement, patient safety, and risk management across all medical and research operations.
- Establish clinical performance metrics and monitor outcomes.
- Hold oversight of consent process and QC across sites.
- Provide oversight of issues identified during monitoring visits looking for trends that need to be shared with other sites or investigations, particularly where there may be a safety issue where additional training or process improvement may be required.
- Ensure adherence to all ICH, GCP, local regulations, study protocols and MCR policies and procedures to maintain participant safety and data integrity.
Business Development & Research
- Identify, assess, and pursue strategic partnerships and funding opportunities with healthcare providers, research institutions, government bodies, and industry stakeholders.
- Provide medical and scientific expertise in the evaluation of potential projects.
- Contribute to business development strategies that position the company as a leader in medical research.
- Provide medical oversight for study design, ethics submissions, and safety monitoring.
- Contribute to the assessment of market opportunities, including risk, feasibility, and alignment with the organisation’s research portfolio.
Stakeholder Engagement
- Work with stakeholders for oversight of all sites to develop additional relationships that would support recruitment.
- Maintain and expand the MCR specialist network.
- Attend Start Up meetings to provide medical input into the recruitment strategy and other areas relevant to the efficient delivery of the study.
- Represent MCR and act as key spokesperson on clinical matters with government health bodies, research partners, industry and community stakeholders.
Budget
- Provide input to budgeting of medical team activities, review and manage medical team utilisation and study realisation across sites.
Communication & Relationships
- Build and sustain strong, trusted relationships across all levels of the organisation and with external stakeholders.
- Communicate with influence and clarity, ensuring alignment of messages with organisational strategy.
- Demonstrates diplomacy, emotional intelligence, and authority in managing complex, sensitive, or high-stakes interactions while ensuring alignment with organisational strategy and values.
Innovation & Continuous Improvement
- Drive a culture of innovation and continuous improvement that enhances organisational performance, efficiency, and value creation.
- Champion new ideas, technologies, and practices that strengthen competitive advantage and service delivery.
- Identify trends across sites and develop new processes and training requirements.
Values & Brand Alignment
- Acts as a role model for organisational values, ensuring they are embedded in decision-making, behaviours, and culture.
- Represents the organisation with integrity, professionalism, and authenticity, strengthening reputation and trust with internal and external stakeholders.
- Aligns leadership actions, communications, and business outcomes with the organisation’s brand promise and cultural identity.
Health, Safety & Wellbeing
- Champions a culture where health, safety, and wellbeing are non-negotiable priorities.
- Ensures compliance with legislation and standards while embedding proactive safety leadership across the organisation.
- Leads by example, creating an environment where all staff feel responsible for safety and supported in their physical and mental wellbeing.
- Balances productivity with the duty of care to protect people and the organisation.
- Maintain overview of Health and Disability Ethics Committee and local regulatory processes.
Qualifications & Experience
- Medical degree with specialist registration.
- Registered with the Medical Board of Australia and Australian Health Practitioner Regulation Agency (AHPRA).
- Significant experience working as a Clinical Trial Investigator and in a senior medical leadership role, preferably in research, healthcare, or pharmaceutical sectors.
- Current ICH GCP Training Certificate
- Deep understanding of clinical governance, ethics, and regulatory compliance.
Personal Attributes
- Demonstrates strategic clinical leadership and vision.
- Exceptional communication and stakeholder engagement skills.
- Strong decision-making, analytical, and problem-solving ability.
Reporting To: Chief Executive Officer
Internal: CEO, Leadership Team, Medical Team, Site Managers.
External: Healthcare providers and industry partners.
Please discuss with Hardygroup Consultant
Service Location: Darlinghurst, Sydney
Annual Budget: Please discuss with HG Principal Consultant
The closing date for applications is Friday, 7th November 2025
The reference number to include in your application is H25_5290
Note: Please use the online platform to submit your application. It will not be accepted via email.
If you require assistance in submitting your application online, please get in touch with Executive Search Coordinator, Aldie Zuñiga: M: +61 (0)49 410 1082 / E: azuniga@hardygroupintl.com
It Is standard practice for HardyGroup to acknowledge receipt of your application no later than the next business day. We request that if you do not receive the acknowledgement, you contact the search coordinator listed above as soon as possible after the 24-hour business period and arrange to resend your application if necessary.
Your application must include:
- 1.Cover letter to the Principal Consultant (Janine Hammat) introducing yourself and addressing the qualifications, requirements and selection criteria;
- 2.An up-to-date copy of your Curriculum Vitae
Please view HG’s Written Application Procedure prior to preparing your documentation and applying for the role.
For a confidential discussion, please contact:

Janine Hammat
HG Principal Consultant
M. +61 (0)488 555 858
E. jhammat@hardygroupintl.com

Lynette Taylor
HG Executive Director, Search & Recruitment
M. +61 (0)431 293 861
E. ltaylor@hardygroupintl.com
ABOUT US
HardyGroup’s (HG) mission is simple
Find and Grow Great Leaders - and we have been doing exactly that for more than 30 years in public and private health, primary, community and aged care as well as the broader public service.
Our synergistic business model of Executive Search and Recruitment integrated with Executive Leadership and Learning is our unique point of difference.
It ensures our clients can count on us for the lifecycle of their organisations leadership journey and why we are regarded as the leading trans-Tasman partner agency by clients.
When engaging HG you can be confident in a deeply personalised experience and service as nothing matters more to us than relationships and results.